Researchers make breakthrough in magnetic geometry-induced quantum geometry and nonlinear transport
2025-06-06SUSTech hosts IOP Forum with President of Institute of Physics Sir Keith Burnett
2025-03-26Researchers discover unusual thermal conductivity in high-symmetry single crystals
2025-03-26New efficient method detects quantum temporal correlations
2025-03-26Researchers explore unconventional magnons predicted by spin space groups
2025-03-25The potential for fuel cells to become the new form of electrical power for vehicles has taken a great leap forward under new research that shows new directions in making them cheaper and longer-lasting.
An interdisciplinary research team at Southern University of Science and Technology (SUSTech) led by Associate Professor Gu Meng (Department of Materials Science and Engineering) and Associate Professor Xu Hu (Department of Physics) have had their paper published in Advanced Science (IF = 15.804). Their article was titled “Sub‐3 nm Intermetallic Ordered Pt3In Clusters for Oxygen Reduction Reaction,” with an examination of ultra-nano-intermetallic compounds.
Fuel cell technology has been an area of considerable focus for researchers due to their high energy density and green credentials. Many countries are investing significant funds into fuel cell technology as part of their broader energy strategies. One of the significant problems with current fuel cell technology is the slow reaction time of the oxygen reduction reaction (ORR) on the cathode. The most prominent commercial catalyst is carbon-supported platinum (Pt / C), which is both scarce and expensive.
Existing research had found that there was an inverse relationship between the size of the catalytic nanoparticles and the volume of low-coordination metal atoms generated – that is, the smaller the particle, the more low-coordination metal atoms were created from the electrocatalytic reaction. These metal atoms could be used as active catalytic sites, and with more of them, there would be reduced costs and increased utilization of those sites. However, there is significantly more surface-free energy, decreasing stability.
The study by the Physics and Materials Science teams examined the creation of ordered intermetallic PtM nanocrystals, integrating Pt with certain metals (M). The group sought to make PtM nanocrystals more stable in acidic and alkaline solutions. Although strategies such as surfactants, salt encapsulation, oxide or polymer coatings provide hope for achieving ordered nanoalloys of the correct size, there had not been any reported research of direct and large-scale fabrication of clustered ordered nanostructures.
An activated loading method prepared a Pt3In (platinum and iridium) ordered cluster catalyst with a catalyst particle size of less than 3 nm. Doping the electronic structure of platinum with iridium reduces Pt’s ability to absorb oxygen, which supports ORR and improves catalytic efficiency. Pt3In is also more stable than previous catalysts with no statistically significant performance degradation after twenty thousand cycles.
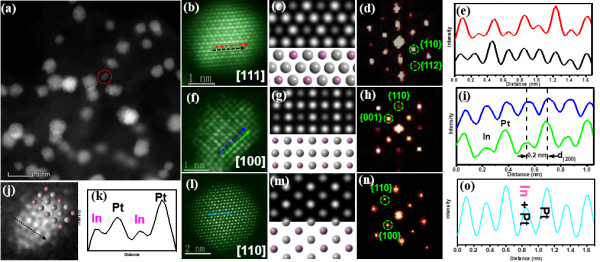
Figure: Electron microscope study of intermetallic compounds
The SUSTech research rejected the use of other reducing agents and surfactants (substances that reduce the surface tension of liquids) while maintaining high yields. The success of their research has shown that their process could be adapted for a wide range of commercial and industrial applications. Their versatile method to obtain ultra-small ordered nano-alloys for catalytic applications like fuel cell catalysts provides practical and reliable research directions for high-performance fuel-cell catalysts.
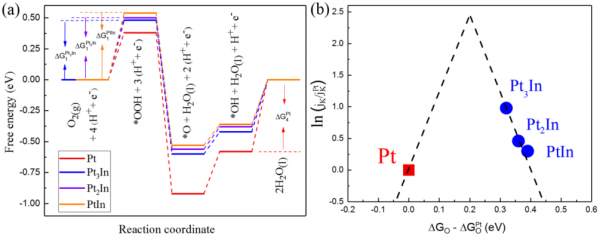
Figure: Relationship between structure and performance of intermetallic alloy
Associate Professors Gu Meng and Xu Hu worked with Professor Sokrates T. Pantelides (Department of Electrical Engineering and Computer Science, Vanderbilt University) and postdoctoral researcher Tianli Feng.
The development and completion of this project were strongly supported by the National Natural Science Foundation of China (NSFC), Shenzhen Clean Energy Research Institute, US Department of Energy, and the McMinn Endowment. Computations at Vanderbilt were completed using computers at the National Energy Research Scientific Computing (NERSC) Center operated by the Department of Energy. The authors acknowledge the assistance of SUSTech Core Research Facilities.
Paper link: https://onlinelibrary.wiley.com/doi/full/10.1002/advs.201901279